
Our high-performance microelectromechanical systems (MEMS) sensors seamlessly integrate into medical devices and applications. As a top MEMS pressure sensor integrator, our experts are here to advance your next breakthrough.
Sensor selection is based on application, size, characteristics, length of use, accuracy and drift requirements. Our OEM Solutions team understands the nuances of each sensor and how to achieve the best results for your device.
Developed over 55 years of innovation, our integration process is guaranteed to improve yields and circumvent MEMS integration challenges, reducing cost and time to market for medical devices.
Viable applications for MEMS pressure sensor integration are virtually limitless, including cardiovascular, airway pressure, intracranial pressure, compartment pressure and urodynamics.
Ultra-miniature and biocompatible, our piezoresistive MEMS sensors fit where space is limited, seamlessly integrating into complex medical devices and delivering decades-proven performance in both invasive and non-invasive care.
Millar’s proven pressure sensing technology can be integrated into an FFR pressure wire or catheter design to enable continuous, high-fidelity measurements within a coronary artery for treatment guidance on intermediate lesions and multi-vessel coronary artery disease (CAD).
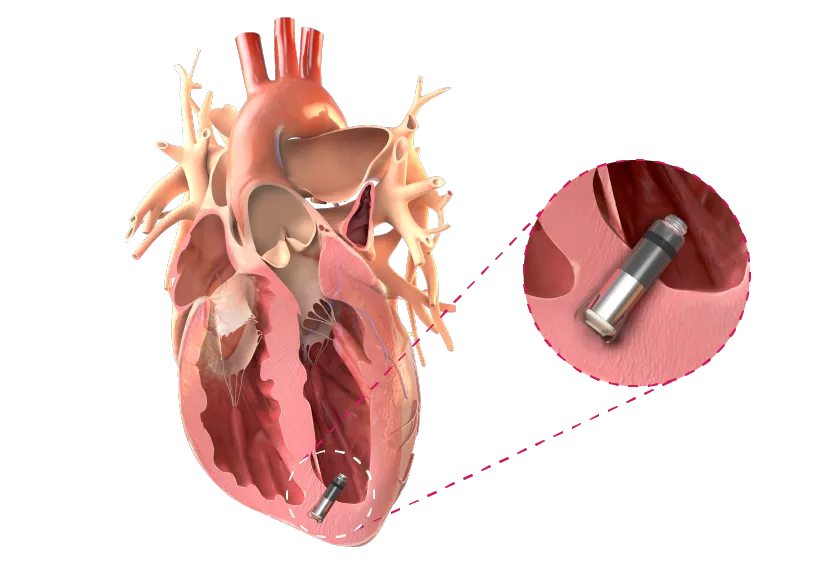
High-fidelity, integrated pressure sensing devices help monitor clinical conditions like pulmonary hypertension, congenital heart disease, mechanical circulatory support, peripheral vascular, left ventricle pressure, valvular disorders and more.

Continuous pressure monitoring in controlled or assisted respiration support devices is critical to reducing complications. The addition of pressure sensors directly to respiratory devices can also support improved feedback mechanisms for intubated patients.

Continuous pressure monitoring for compartment syndrome is critical in understanding pressure changes over time. MEMS pressure sensing technologies provide stability to deliver reliable, accurate measurements even during patient movement.
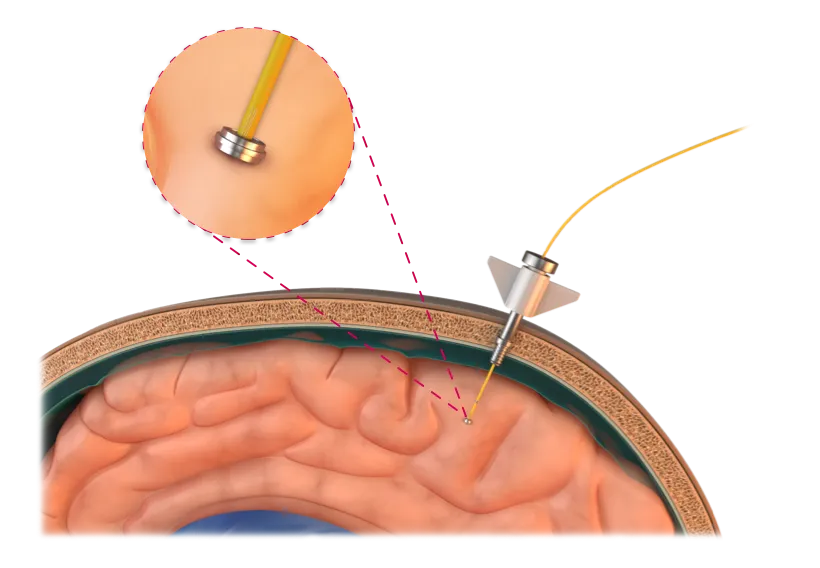
Millar’s MEMS pressure sensors can be integrated into minimally invasive devices to provide continuous intracranial pressure (ICP) monitoring, helping clinicians manage traumatic brain injury, hydrocephalus and other neurocritical conditions with precision.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Suspendisse varius enim in eros elementum tristique. Duis cursus, mi quis viverra ornare, eros dolor interdum nulla, ut commodo diam libero vitae erat.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Suspendisse varius enim in eros elementum tristique. Duis cursus, mi quis viverra ornare, eros dolor interdum nulla, ut commodo diam libero vitae erat.

MEMS stands for microelectromechanical system. “Micro” means these devices typically measure less than a millimeter in any direction. They are “systems” in that they employ both electrical and mechanical forces in a single unit.
Millar pressure transducers are the gold standard for accurate measurement of pressure in clinical and pre-clinical applications. Throughout Millar’s history, the company has developed progressively smaller sensors both to minimize interference with physiological parameters and to access smaller venues. Miniaturization to the level of MEMS devices opens up new opportunities for acquisition of pressure information in greater detail and from formerly inaccessible areas.

| PFB1100 Full Bridge | PFB1000 Full Bridge | PFB800 Full Bridge | PFB440 Full Bridge | PHB225 Half Bridge | |
|---|---|---|---|---|---|
| Circuit | 4 MEMS piezoresistors | 4 MEMS piezoresistors | 4 MEMS piezoresistors | 4 MEMS piezoresistors | 2 MEMS piezoresistors |
| Sensitivity | 8–15 µV/V/mmHg | 8–15 µV/V/mmHg | 8–15 µV/V/mmHg | 8–15 µV/V/mmHg | 5 µV/V/mmHg |
| Pressure Range | -300 to +1000 mmHg | -300 to +1000 mmHg | -300 to +1000 mmHg | -300 to +1000 mmHg | -50 to 300 mmHg |
| Accuracy & Linearity | < 1% error over -50 to 200 mmHg applied pressure | < 1% error over -50 to 200 mmHg applied pressure | < 1% error over -50 to 200 mmHg applied pressure | < 1% error over -50 to 200 mmHg applied pressure | ±1% error over -50 to 150 mmHg applied pressure |
| Temperature Error | < 1% (15–40) deg C. | < 1% (15–40) deg C. | < 1% (15–40) deg C. | < 1% (15–40) deg C. | < 3.5% (15–40) deg C. |
| Drift | Average = 0.2 ± 0.1 mmHg over 3 hours | Average = 0.2 ± 0.1 mmHg over 3 hours | Average = 0.2 ± 0.1 mmHg over 3 hours | Average = 0.2 ± 0.1 mmHg over 3 hours | Average = 0.89 ± 0.44 mmHg over 7 days |
| Bridge Resistance | 3.9K to 5.1K | 3.9K to 5.1K | 3.9K to 5.1K | 3.9K to 5.1K | 3.4 KΩ ± 20% |
| Resolution | Analog output | Analog output | Analog output | Analog output | Analog output |
| Sensor Type | Vented | Vented | Vented | Vented | Absolute |
MEMS pressure sensors make use of piezoelectricity. Pressure applied to certain materials produces an electrical voltage, which can be measured. MEMS piezo-resistive sensors sense a change in resistance across a thin silicon diaphragm. This technology is widely used in industrial sensors and is well proven. The sensors are rugged and measure pressure only at the source. Over the years, Millar has perfected integration methods with these sensors to greatly reduce the effects of drift resulting from integration. Catheters built with sensor tips are robust and can navigate tortuous paths in the human vasculature. The interface electronics are fairly simple and can connect to commercially available monitors. Since pressure is measured only at the tip, this technology does not suffer from damping and resonance effects of fluid-filled lines.
Millar MEMS sensors offer faster response, higher fidelity and fewer artifacts than fluid-filled transducers, which can be affected by damping and motion-induced errors.
Click here to read more about the difference between fluid-filled sensors and MEMS sensors.
We offer a diverse range of sensor sizes for integration, catering to various medical applications. Our sensors span in size from 1F (French) to 3F (French), accommodating a wide spectrum of medical device requirements.
The integration process of these sensors is a meticulous and collaborative endeavor. Our comprehensive approach involves several steps, including assessment, consultation, sensor selection and more. To learn more about the integration process and how Millar can assist you, contact us today. To see all our range of sensor solutions, download our sensor portfolio.
Yes. Millar offers implantable MEMS pressure sensors. For acute monitoring, our catheter-based solutions support days to weeks; for chronic monitoring, our TiSense™ implantable platform is engineered for 5+ years in-body use (with appropriate system design). Typical applications include cardiovascular, neurocritical care and more.
Millar offers both analog differential voltage as well as digital I²C output options.
MEMS sensors offer high accuracy (< ±3% over –30 to +300 mmHg) and superior stability, with maximum drift < 6 mmHg in 12 hours.
Yes, you can request a Pressure R&D Kit to bench-test sensors in your prototype before full integration. Explore our R&D kits here.
Millar’s MEMS sensors are used in diverse applications including cardiovascular (FFR, ventricular), airway monitoring, intracranial pressure, compartment pressure, and more.
Ready to learn more about our acute pressure sensors? Our medical sales team is here to answer your questions and help you explore how we can support your clinical or research goals.

Accelerate your medical device development with our comprehensive pressure R&D Kits. These kits are equipped with the sensors you need to quickly evaluate and integrate our technology into your medical application.
Millar’s patented strain-gauge technology in the Codman Microsensor ICP Transducer gives medical practitioners the precise, reliable information they need to intervene quickly and relieve brain-damaging pressure.