
During the discovery phase, we help determine the best pressure sensor integration solution for your medical device. Leveraging our extensive pressure sensing technology expertise, this initial phase identifies potential issues early in the development process, eliminates unnecessary costs and delays, and provides insight into project scope and future manufacturing requirements.

Once a project passes through discovery, it moves into the proof-of-concept phase, which involves product customization to test full sensor integration into one or more working designs. The Millar OEM team explores multiple design iterations before selecting and refining a final design, then develops initial documentation.

Millar supports early-stage prototyping in our ISO 13485-certified manufacturing facility and ISO Class 7 clean room. Our goal at this stage is to establish a stable design, complete key design control documentation, and define production volume requirements.

Millar’s manufacturing development phase defines the critical processes that impact a product’s overall cost and successful production. Our manufacturing engineers will begin process validation work and review the designs and components to determine the product’s ease of fabrication, possibly suggesting modifications to maximize production yields.

As your device progresses beyond prototyping, Millar prepares it for real-world readiness. Our engineering and production teams collaborate to develop pilot builds, conduct design verification, and ensure readiness for regulatory testing and clinical trials. With full-scale manufacturing capabilities, including our ISO 13485-certified facility with an ISO Class 7 clean room, we’re equipped to produce sensor-integrated components that meet the highest standards for medical devices.

Once your device is validated for performance and safety, it advances to clinical trials. Millar supports this critical phase with production-quality builds, thorough documentation and regulatory expertise — ensuring your device meets the stringent requirements for human use and is ready for real-world impact.

Millar is your OEM partner every step of the way. Once your medical device is in commercial production, we provide sustaining engineering services to ensure production lines are optimized. Services include general maintenance, process improvements, and a dedicated product engineer to facilitate any future changes or challenges.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Suspendisse varius enim in eros elementum tristique. Duis cursus, mi quis viverra ornare, eros dolor interdum nulla, ut commodo diam libero vitae erat.

.webp)
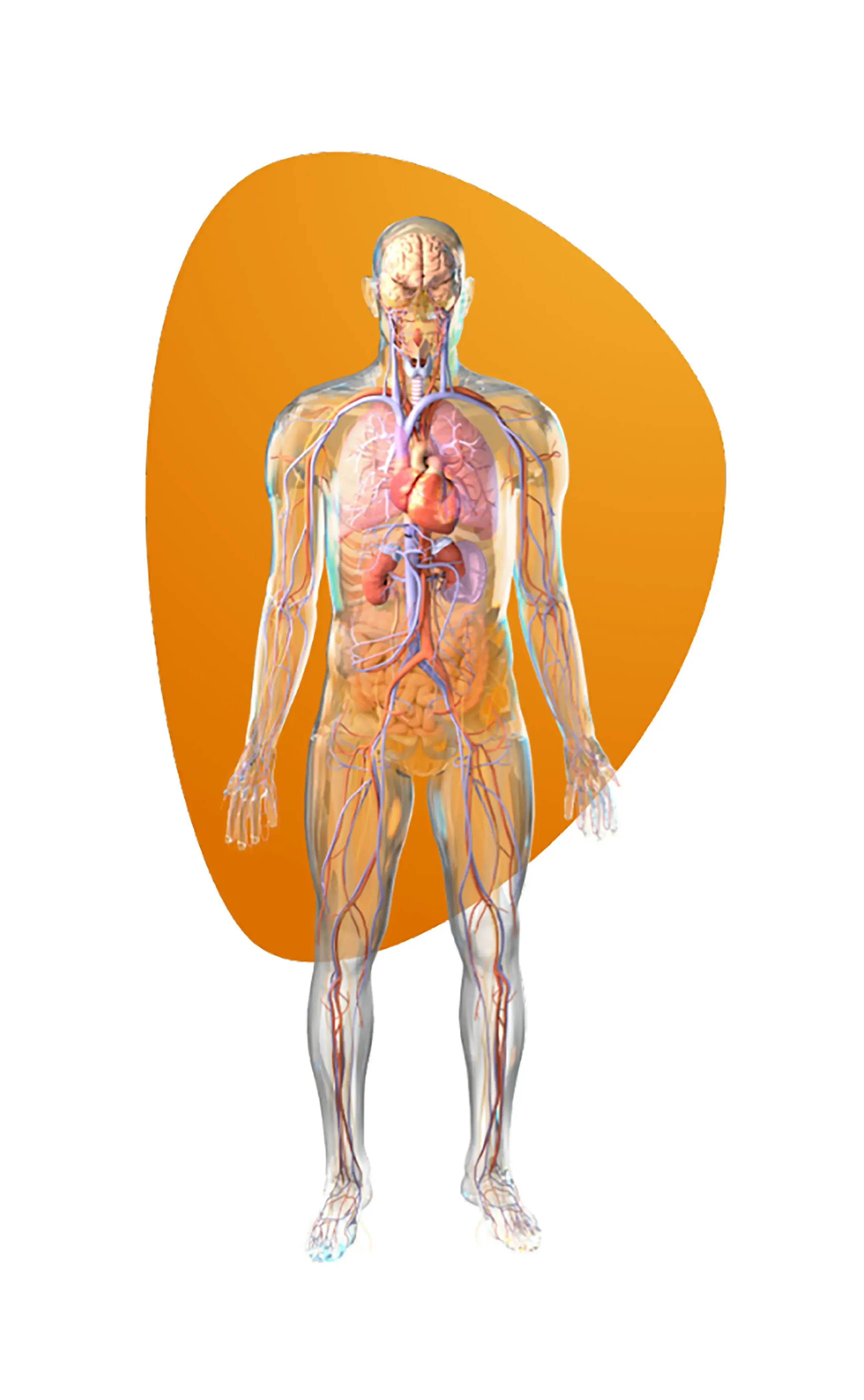

Key applications include heart failure, pulmonary hypertension and ventricular assist devices.
Key applications include traumatic brain injuries and hydrocephalus.
Key applications include smart pills and GI motility studies.
Key applications include smart bandages and chronic wound assessment.
Key applications include pulmonary pressure monitoring, esophageal pressures and sleep apnea diagnostics.
Key applications include salivary pH monitoring and bite force analysis.
Key applications include bladder pressure, flow sensing and urodynamic studies.
Proper integration of a MEMS pressure sensor in a medical device requires in-depth knowledge of sensor types, capabilities, validation and manufacturing requirements.


