
From pressure and pH to multi-parameter sensing, our R&D kits give you the tools to validate, benchtop test and push boundaries.

Our MEMS R&D Kit is an essential starting point for OEMs looking to integrate Millar’s high-fidelity MEMS pressure sensors into their devices. The customizable kit gives you everything you need to benchtop test and perform thorough performance evaluations. Together with our expert support, this kit helps identify integration challenges early and streamline development from the start.
Kit contents:
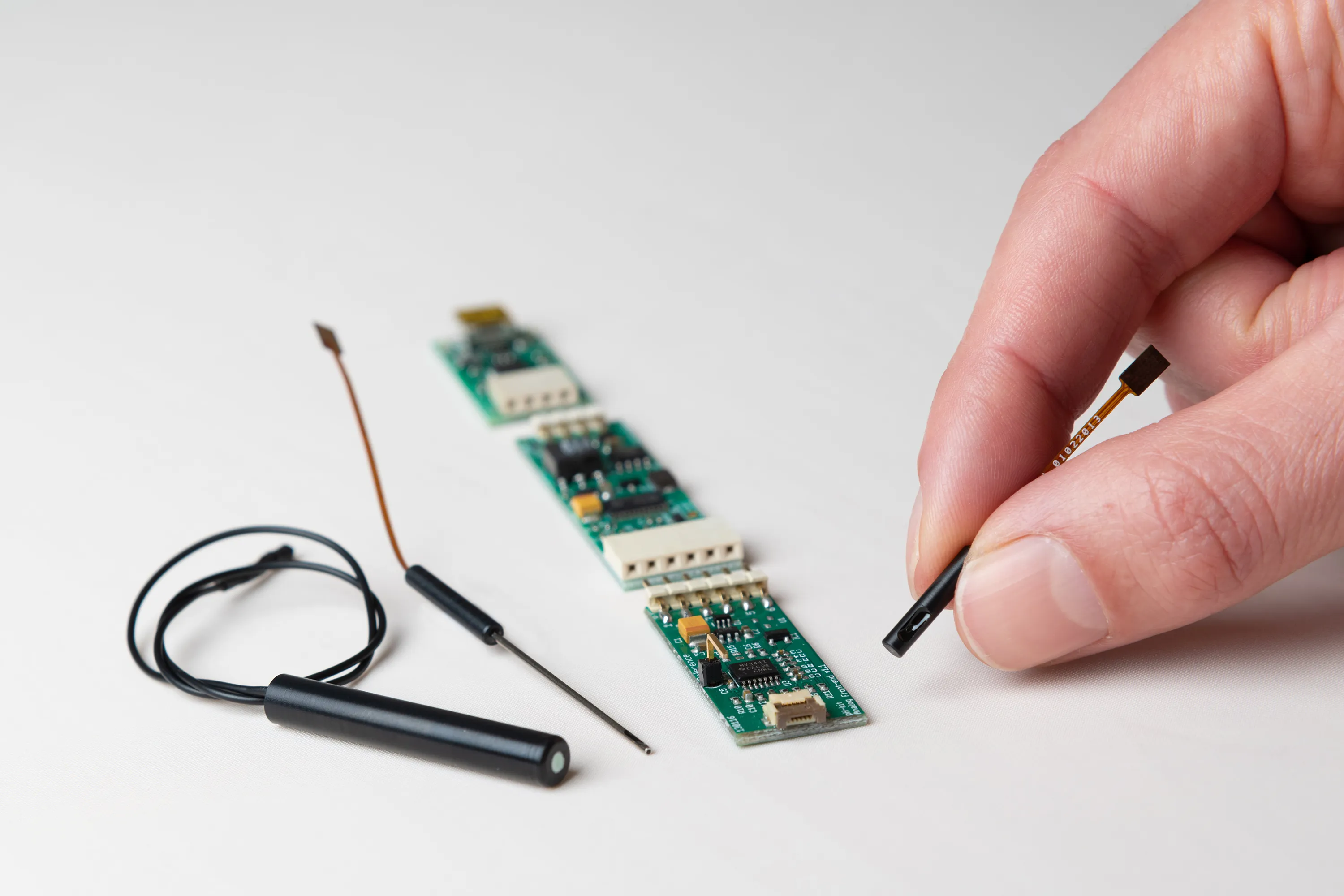
Millar offers a glass-free modular R&D evaluation pH kit designed specifically for development and testing purposes. With this ISFET pH kit, development engineers and researchers have a large degree of flexibility in how they integrate our proprietary ISFET pH sensor into their applications or experimental set-ups. Due to the modular design, the functionality can be expanded as required and components can be replaced individually. The ISFET pH sensor module, the reference electrode module and the Analog Front-end module are always needed as a basis for pH measurement.
Kit contents:
Clinical-Grade Reliability: Backed by Millar’s decades-long leadership in pressure sensor innovation and quality.
Expert Support: Access to engineering support and technical documentation for seamless integration.
Modular and Scalable: Start with a demo, perform your own benchtop testing, and scale into your device roadmap with confidence.


“Partnering with Millar has really helped us understand what is needed to take our devices to the next level. Being able to benchtop-test their sensors in our device prototypes was very insightful and helped us get to know their sensor technology.”
— Millar OEM Partner

