In the ever-evolving landscape of healthcare technology, chronic pressure measurement plays a pivotal role in monitoring and managing various medical conditions, particularly chronic heart failure. Two cutting-edge platforms, TiSense and CardioMEMS, have emerged as leaders in this field, offering innovative solutions that empower both patients and healthcare providers. In this blog, we'll explore a comparison of these two platforms, highlighting their similarities, and differences, and why TiSense is the best choice for companies seeking to integrate a chronic pressure sensor into their medical devices.
Discovering TiSense
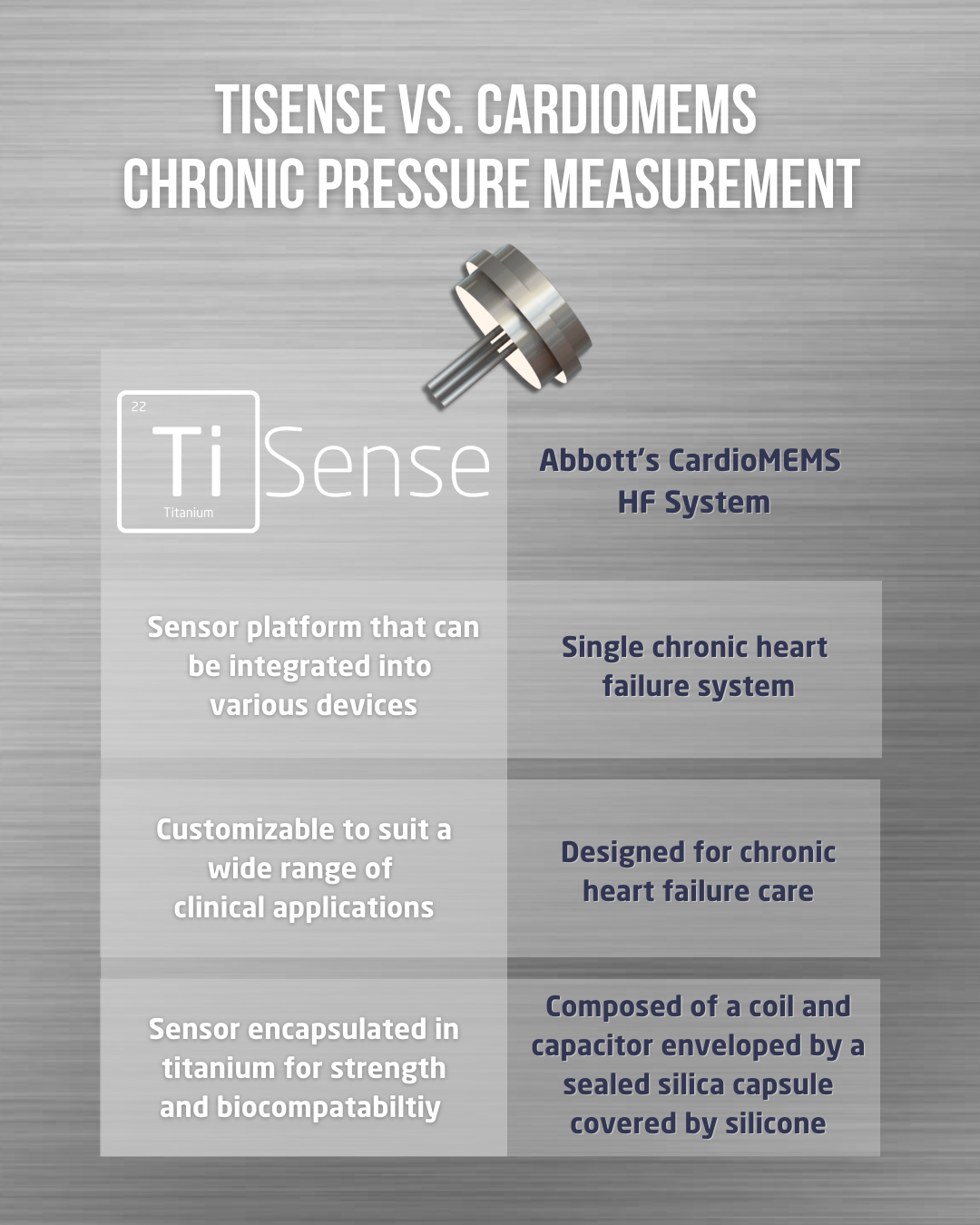
TiSense is a groundbreaking chronic pressure measurement platform. This innovative technology integrates a MEMS piezo-resistive sensor into a chronic implantable sensor module constructed from titanium. TiSense's standout feature is its long-term implantation capability of >5 years, providing uninterrupted monitoring without the need for frequent sensor replacements. Additionally, TiSense is highly adaptable, catering to a wide range of medical applications and devices, making it an ideal choice for companies seeking versatile, long-term pressure measurement solutions for their devices.
About CardioMEMS
CardioMEMS is a pioneering system designed to revolutionize heart failure care. It features a small implantable sensor that monitors pressure changes within the body, specifically in the pulmonary artery. This remote monitoring platform is an FDA-approved device for both Class II and Class III heart failure patients. CardioMEMS has been proven to reduce hospitalizations and improve outcomes, making it a valuable tool for managing heart failure patients' health more effectively.
Comparing the Two Platforms
TiSense and CardioMEMS share several similarities in their approach to chronic pressure measurement. Both platforms leverage state-of-the-art MEMS sensor technology to continuously monitor pressure changes within the body, particularly in the context of heart failure. Additionally, they offer the convenience of remote monitoring, enabling healthcare teams to receive real-time data for proactive treatment adjustments. TiSense, in particular, stands out with its potential for real-time adjustments to the integrated device, enhancing its versatility.
However, where these platforms differ lies in their device styles and application adaptability. TiSense is a highly adaptable solution, boasting application agnosticism that allows seamless integration into a wide array of medical contexts. Its versatility extends to implantable MEMS sensors that can be incorporated into various devices, offering diverse opportunities for innovation. In contrast, CardioMEMS, while FDA-approved and effective in heart failure management, is less versatile and tailored for this specific medical application. TiSense's ability to cater to dynamic and static pressure monitoring needs in various scenarios positions it as a forward-thinking choice for those seeking a versatile, long-term pressure measurement solution in the ever-evolving landscape of healthcare technology.
Why Choose TiSense?
TiSense stands out as the superior choice for companies seeking to integrate a chronic pressure sensor into their medical devices due to its unparalleled combination of long-term implantation capabilities and application versatility. With TiSense, companies can offer their customers a cutting-edge solution, ensuring continuous monitoring without the risks of frequent replacements. Furthermore, TiSense's application agnosticism allows it to adapt seamlessly to various medical contexts, from dynamic cardiovascular pressure monitoring to static intracranial or intraocular pressures. This flexibility empowers companies to provide tailored solutions that cater to diverse healthcare scenarios, ultimately enhancing the performance and reliability of their medical devices.